 Republished with permission of Momentum,
Republished with permission of Momentum,
a School of Engineering electronic publication.
 The Research Experience for Undergraduates (REU) program provides undergraduates with exposure to a stimulating research environment. The students participating in the REU program had the opportunity to present their work during the July 26 Innovation Connection academic/industry networking event hosted at Nerac in Tolland and co-sponsored by Nerac and OpenSky. Nerac president Kevin Bouley, who hosts a number of UConn start-ups in his Tolland facility, noted “This event showcases the collaborations between students, faculty and the private sector. It was very interesting to see RPM Sustainable Technologies participate, given that they are located in the Nerac building as a launching pad for their commercial enterprise.”
The Research Experience for Undergraduates (REU) program provides undergraduates with exposure to a stimulating research environment. The students participating in the REU program had the opportunity to present their work during the July 26 Innovation Connection academic/industry networking event hosted at Nerac in Tolland and co-sponsored by Nerac and OpenSky. Nerac president Kevin Bouley, who hosts a number of UConn start-ups in his Tolland facility, noted “This event showcases the collaborations between students, faculty and the private sector. It was very interesting to see RPM Sustainable Technologies participate, given that they are located in the Nerac building as a launching pad for their commercial enterprise.”
Before an audience of entrepreneurs, small business gurus, state government officials, IP experts, faculty and members of the investment community, each young researcher/entrepreneur delivered a two-minute “elevator pitch” presentation of his/her work and then spoke in greater detail with attendees during the informal networking event. The forum enabled the students to test their mettle in the real-world situation faced by entrepreneurs every day.
While all REU programs entail scholarly research, this innovation-oriented REU requires the students to participate in a business and entrepreneurship seminar taught by professor Richard Dino of the School of Business. Furthermore, the students’ research was co-sponsored by commercial businesses – a novel twist that underscores the commercial intent of the research challenges they addressed while working in the UConn faculty laboratories.
The REU theme was conceptualized by Dr. Jeffrey McCutcheon, assistant professor of Chemical & Biomolecular Engineering, and Entrepreneur-in-Residence Robin Bienemann, and NSF began funding the project in 2012. In his introductory remarks to the audience, Dr. McCutcheon explained the genesis of the Innovation REU and noted that his goal was to “introduce the students to applied science and the way products make it to market.”
The eight innovation REU students and their projects are summarized below.
 Joseph Amato (Univ. of Minnesota – Twin Cities) researched reactive spray deposition technology for the one-step production of catalysts and electrodes in fuel cells. His research aim was to improve the efficiency of proton exchange membrane (PEM) fuel cells for the fuel cell and fuel-cell automotive markets. Sponsor: Proton OnSite; faculty mentor: Dr. Radenka Maric (Chemical & Biomolecular Engineering). Poster.
Joseph Amato (Univ. of Minnesota – Twin Cities) researched reactive spray deposition technology for the one-step production of catalysts and electrodes in fuel cells. His research aim was to improve the efficiency of proton exchange membrane (PEM) fuel cells for the fuel cell and fuel-cell automotive markets. Sponsor: Proton OnSite; faculty mentor: Dr. Radenka Maric (Chemical & Biomolecular Engineering). Poster.
Isaac Batty (California State Univ. – Long Beach) researched bio-oil production from the fast catalytic pyrolysis of lignocellulosic biomass (trees). His objective was to investigate the effect of temperature and various catalyst/biomass ratios on the quality of bio-oil produced from biomass. Sponsor: W.R. Grace & Co.; faculty mentor: Dr. George Bollas (Chemical & Biomolecular Engineering). Poster.
Ryan Carpenter (Univ. of Buffalo)designed an experimental apparatus enabling researchers to observe the antimicrobial susceptibility of multispecies biofilms. Biofilms are common (e.g., dental plaques) and often contain multiple species of bacteria such as Staphylococcus aureus. Biofilms are a costly problem for many industries, including food processing, oil recovery and medical implant operations. Sponsor: BASF; faculty mentor: Dr. Leslie Shor (Chemical & Biomolecular Engineering). Poster.
William Hale (UConn) sought to understand whether acetate and butyrate influence the anaerobic fermentation of waste glycerol – a byproduct from biodiesel production – into 1,3-propanediol. 1,3-propanediol is used in the manufacture of polyesters, solvents, lubricants and other products. Sponsor: RPM Sustainable Technologies; faculty advisor: Dr. Richard Parnas (Chemical & Biomolecular Engineering). Poster.
Justine Jesse (Univ. of Massachusetts) researched heat treatments that produce the strongest possible electrospun nanofibers, used in water filtration and industrial plants, without compromising performance. Sponsor: KX Technologies; faculty mentor: Dr. Jeffrey McCutcheon (Chemical & Biomolecular Engineering). Poster.
Kyle Karinshak (Univ. of Oklahoma) researched the photocatalytic degradation of a specific fluorescent dye in aqueous environments through the use of a titanium oxide/metal doped catalyst. Kyle found titanium oxide/metal-doped fly ash to be an effective catalyst enabling the degradation of the dye, which is released from textile plants and inhibits the passage of sunlight through water/ Sponsor: VeruTEK Corp.; faculty mentor: Dr. Steven Suib (Chemistry; Institute for Materials Science). Poster.
Zachariah Rueger (Iowa State Univ.) sought to maximize the specific surface area of activated carbon nanofiber nonwoven mats, which are used in water purification and for electricity generation in certain fuel cells. A greater surface area allows greater volumes of wastewater to be purified quickly. Sponsor: KX Technologies; faculty mentor: Dr. Jeffrey McCutcheon (Chemical & Biomolecular Engineering). Poster.
Kyle Stachowiak (Vanderbilt Univ.) researched techniques to optimize the atomic layer deposition of copper on a component, the rectenna, used to enhance the performance of solar cells. A rectenna collects solar radiation and converts it to usable energy. Techniques for applying copper more reliably will improve the efficiency of solar cells. Sponsor: Scitech Associates LLC; faculty mentor: Dr. Brian Willis (Chemical & Biomolecular Engineering). Poster.
 The University of Connecticut Chemical & Biomolecular Engineering undergraduate students recently attended the AIChE 2013 Annual Meeting in San Francisco. The AIChE Annual Meeting is an educational forum for chemical engineers focused on research, growth, and innovation. Industry and academic professionals discussed a variety of topics relating to new research, technologies, and studies in chemical engineering.
The University of Connecticut Chemical & Biomolecular Engineering undergraduate students recently attended the AIChE 2013 Annual Meeting in San Francisco. The AIChE Annual Meeting is an educational forum for chemical engineers focused on research, growth, and innovation. Industry and academic professionals discussed a variety of topics relating to new research, technologies, and studies in chemical engineering.
 Mr. Shoucheng Du, Prof. Julia Valla, and Prof. George Bollas are making exciting progress in developing the process of biomass catalytic pyrolysis. Their recent achievements are published in Green Chemistry (
Mr. Shoucheng Du, Prof. Julia Valla, and Prof. George Bollas are making exciting progress in developing the process of biomass catalytic pyrolysis. Their recent achievements are published in Green Chemistry (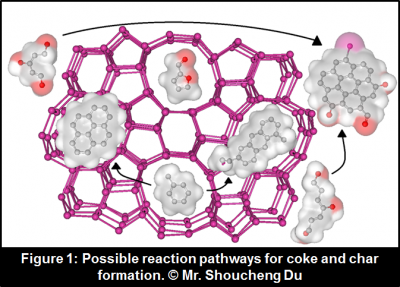

 The Research Experience for Undergraduates (REU) program provides undergraduates with exposure to a stimulating research environment.
The Research Experience for Undergraduates (REU) program provides undergraduates with exposure to a stimulating research environment.
 The chemical engineering graduate program at the University of Connecticut is comprised of bright, innovative leaders who are motivated by change and challenge. The program offers the opportunity for students to enhance their skills and develop their potential.
The chemical engineering graduate program at the University of Connecticut is comprised of bright, innovative leaders who are motivated by change and challenge. The program offers the opportunity for students to enhance their skills and develop their potential.

 Dr. Nieh was recently awarded a National Science Foundation grant in 2012 to design such nano-carriers. “Lipid-based nanodiscs and vesicles have the potential to serve as delivery carriers for therapeutics or diagnostic agents, so the stability of the structure is an important issue,” he said.
Dr. Nieh was recently awarded a National Science Foundation grant in 2012 to design such nano-carriers. “Lipid-based nanodiscs and vesicles have the potential to serve as delivery carriers for therapeutics or diagnostic agents, so the stability of the structure is an important issue,” he said.
 Dr.
Dr. 